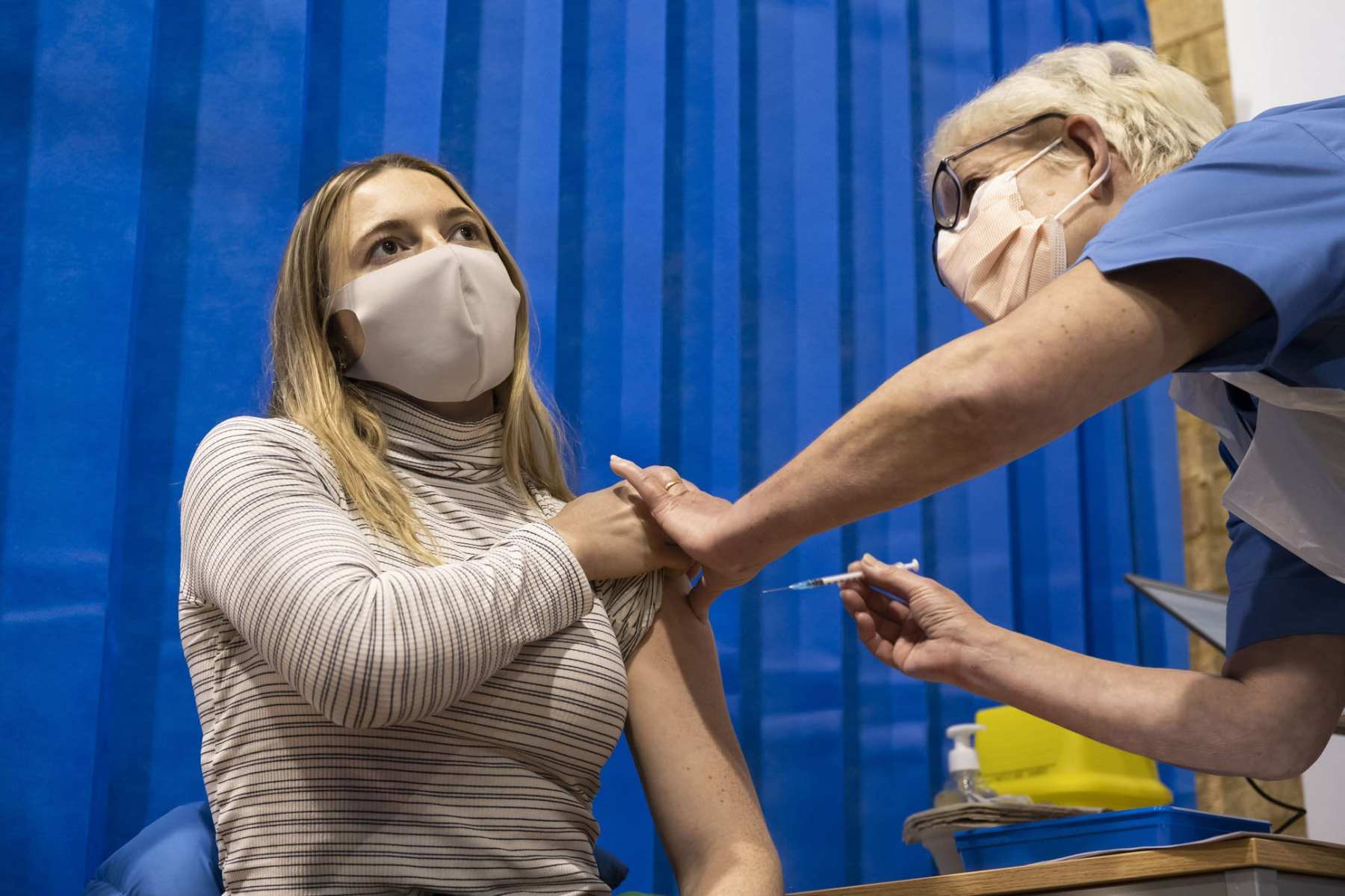
A top government panel voted in favor of granting an emergency use authorization for Pfizer’s coronavirus vaccine candidate for people 16 and older, including those who are pregnant — a major step toward administering the first round of COVID-19 immunizations in the United States, and a notable departure from how British regulators addressed the pregnancy question.
The Vaccines and Related Biological Products Advisory Committee’s recommendation will now head to the Food and Drug Administration, which is expected to accept that advice. If the FDA grants emergency authorization, some Americans could start receiving vaccines within a matter of days, and the majority will likely be women.
Emergency use authorizations (EUAs) are an interim way to make drugs available in crises, but they require less safety and efficacy data than does full government approval. At Thursday’s meeting, government scientists emphasized the need to track the health of people who receive the initial Pfizer vaccines, monitoring both their level of immunity and what kind of adverse reactions they have. Pfizer is planning to seek full government approval next spring, after submitting more vaccine data.
Still, the authorization has major implications. Last week, the Centers for Disease Control and Prevention (CDC) advised that the first doses of COVID-19 vaccines be given to health care workers — particularly those in direct contact with people who have contracted the coronavirus — and residents of nursing homes. State governments will be charged with distributing vaccines to eligible residents. But there won’t immediately be enough vaccines to cover everyone, even in those groups.
Women will likely constitute the majority of immediate beneficiaries. About 20 million Americans work in health care, and 75 percent of those workers are women. More than two-thirds of the nation’s 1.3 million long-term nursing home residents are women.
Notably, the FDA advisory committee did not explicitly prohibit giving the vaccine to pregnant people otherwise eligible for the vaccine. That leaves open the door for health care workers who are pregnant and breastfeeding during the initial vaccine distribution — an estimated 330,000 people — to get the two-shot regimen when their states and hospitals receive the vaccine. Pfizer’s large-scale vaccine trial excluded those who were pregnant or breastfeeding.
“We have really no data to speak to risks specific to pregnant women or fetuses, but also no data that would warrant a contra-indication to use in pregnancy at this time,” Doran Fink, an FDA deputy director who specializes in vaccines, said at the meeting. “In the interest of allowing women of child-bearing potential who would consider vaccination, who are in the population approved for use under an EUA – they would be free to make their own decision, in conjunction with their health care providers.”
During the meeting, government officials acknowledged the particular challenge posed by vaccinating pregnant people, especially those who are more likely to be exposed to the virus through work.
“The early indication is there may be a higher risk of preterm delivery for pregnant women infected with COVID-19 compared to women without COVID-19, but there’s ongoing efforts to assess those and other pregnancy-related risks,” Aron Hall, a CDC scientist, told the panel.
On Thursday, Pfizer said it expected to have data by mid-December about whether animal trials of the vaccine would have negative repercussions on the reproductive system — something known as “reproductive toxicity” — which is an important question for pregnant people taking the vaccine.
And though Pfizer excluded pregnant people from enrolling in the study, 23 participants became pregnant during the trial, according to company data. Twelve received the vaccine, and 11 were in the placebo group.
That information isn’t enough to draw larger-scale conclusions. The FDA has required that Pfizer track and submit regular data about how people respond if they get the vaccine while pregnant, including whether they develop any adverse events. The company also intends to launch studies on pregnancy and the vaccine next year.
Immediately, it’s likely that states and local health care systems will have to decide how to distribute a limited supply of vaccines. They could determine on their own whether to treat pregnant health care workers as part of the immediate priority immunization group.
Medical groups, including the Society for Maternal-Fetal Medicine and the American College for Obstetricians and Gynecologists, have argued that pregnant health care workers should be given the option of taking the vaccine. The technology behind Pfizer’s vaccine, messenger RNA, isn’t known to cause special problems for either pregnant people or their fetuses. (Messenger RNA is also the basis for the Zika vaccine.)
In an op-ed published this week at Stat News, three medical ethicists argued that pregnant health care workers should be given the option to take any early vaccine, factoring in their risk of on-the-job COVID exposure.
“A restrictive policy would not only deny pregnant and lactating individuals the opportunity to work with the same protection afforded their co-workers, but would further strain available human health resources over the next few months, when pandemic cases — and the need for health care — are peaking,” the authors wrote.