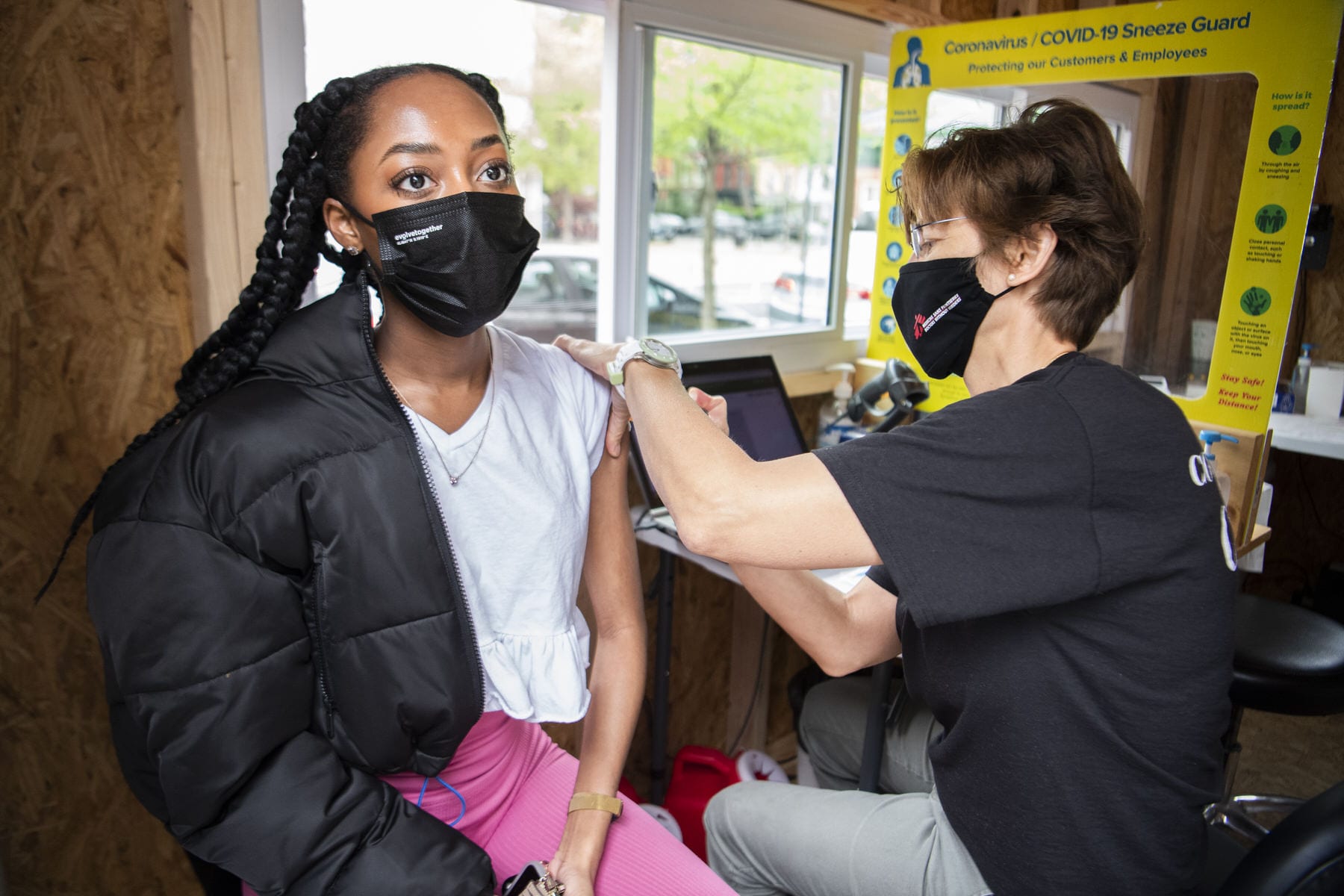
Jessi Healey had been looking forward to receiving her Johnson & Johnson one-dose coronavirus vaccine on Tuesday. She scheduled the shot last week, on the first day that the 37-year-old was eligible to get vaccinated in North Carolina.
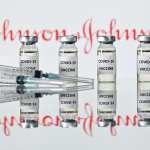
On the same day Healey was set to get her vaccination, the federal government recommended a pause on Johnson & Johnson vaccines after six women developed blood clots and low blood platelet levels within two weeks of taking the shot — advice vaccine providers are already taking up.
The condition is exceedingly rare. More than 6.8 million people have gotten the Johnson & Johnson vaccine so far, making the chance of having developed a clot less than 1 in a million. Every case occurred in a woman between ages 18 and 48, sometimes between 6 and 13 days after vaccination.
Healey, a self-employed social media manager who lives in a suburb of Charlotte, woke up to the news and felt frustrated. She had spent hours online looking for a vaccine shot, and as appointments were snatched up in real time as she tried to book, she finally secured a Johnson & Johnson shot. Just a few hours before her Tuesday afternoon appointment, staff at the pharmacy where Healey was scheduled to visit called her to cancel.
“My frustration is not with the precaution, or even the hassle,” she said. “It’s more just that I want to get the vaccine. A vaccine.”
Healey is now back to looking. Staff at the pharmacy said they didn’t have any appointments available, so she’s checking her options online. Healy is the only adult in her household who has yet to get a shot, and it’s weighing on her as she tries to make plans with her family.
A top advisory committee to the Centers for Disease Control will meet Wednesday to discuss the data on the blood clots. The pause is expected to last only a few days, Food and Drug Commissioner Janet Woodcock told reporters Tuesday. The Johnson & Johnson vaccines make up a fraction of the 190 million COVID-19 vaccine doses so far administered in the United States.
The pause is recommended out of an “abundance of caution,” government officials stressed.
“This is important, in part, to ensure that the health care provider community is aware of the potential for these adverse events and can plan for proper recognition and management due to the unique treatment required with this type of blood clot,” Dr. Anne Schuchat, principal deputy director of the Centers for Disease Control and Prevention and Dr. Peter Marks, director of the FDA’s Center for Biologics Evaluation and Research, said in a statement.
Government officials said they are communicating with local vaccine providers to make sure people who had appointments for Johnson & Johnson vaccines get rescheduled imminently with other vaccines.
“This may be a bit bumpy,” Schuchat said in a press conference. “We want to make sure people who had been scheduled for vaccination are able to get that.”
The issue involves cerebral venous sinus thrombosis, a blood clot that is very rare and requires different treatment from other kinds of blood clots. It appears similar to a blood clot seen in rare cases with the AstraZeneca vaccine, which is being used in many other countries but not yet in the United States. Some countries have temporarily paused the use of the AstraZeneca shot, but public health experts have stressed that the rarity of developing a blood clot does not outweigh the immense benefit of getting a vaccine.
Some researchers worry that pausing the Johnson & Johnson shot could make people more hesitant about taking a coronavirus vaccine — and not just Johnson & Johnson’s.
“The problem is people are going to look at this and not dig into any of the other stuff. They’re going to be like, ‘People don’t know what they’re doing,’ and not get the vaccine,” said Rupali Limaye, an associate scientist at Johns Hopkins University, who studies vaccine hesitancy. “I think people will take this experience and apply it to anything that comes on the market.”
Women interviewed by The 19th expressed concern — some about their own health, and others for the wider implications the news could have on general vaccine uptake and accessibility.
Megan Parcell, a mother of two in Indianapolis, got the Johnson & Johnson shot last week at a local CVS pharmacy. The news on Tuesday rattled the account executive. Although the 35-year-old hasn’t experienced any serious symptoms related to her shot, she said her family has a history of blood clots. She would have actively sought another vaccine had she known about the potential risk. She is considering reaching out to her doctor as a precaution.
“I keep telling myself like, ‘OK. It’s six individuals as of right now, like six out of the millions that have gotten it.’ So I’m trying to figure out how I’m going to cope with my anxiety for the next week until I get out of that 13-day window.”
Though all six cases have appeared in women, the sample is too small to determine whether certain groups of people were at greater risk for blood clots, government officials said. More blood clotting cases may be identified in coming days, though Marks said he hopes the number is small.
Oral hormonal contraception can also elevate the risk for blood clotting by between .3 percent and 1 percent over a decade. But so far, though all women who developed clots were of reproductive age, “it’s not clear that there’s any association with oral contraceptive pill birth control,” Marks said.
Unlike the Pfizer and Moderna vaccines, the Johnson & Johnson shot was tested in areas that included the more transmissible coronavirus variant first seen in South Africa. Slowing down use of that vaccine — or eroding vaccine confidence — could be particularly damaging, Limaye worried.
Because the vaccine only requires one dose it has also been touted as particularly effective in reaching people who might not have the time and flexibility to easily schedule two shots. Unlike the Pfizer and Moderna vaccines, it does not require freezer storage, which had also made it potentially more viable in rural areas, or for use in mobile clinics.
Jangela Shumskas got her Johnson & Johnson shot last Wednesday at a community center in Washington, D.C. The 44-year-old consultant, who posted a video of herself getting the shot on social media, said she is not panicking about the news because of the statistical rarity of the cases. She said she’ll monitor for any additional cases, but she’s more concerned about the implications for others. She specifically scheduled a Johnson & Johnson shot to show others it is a safe option.
“I feel kind of remorseful that one of the most promising vaccine candidates that we had available to kind of get to lower-income communities — and to households that are less able to make it to two appointments and to individuals and households that need immunogenicity sooner rather than later — this is a big blow,” she said.
Shumskas added that she is concerned about the ramifications of the pause on women who had been eligible for the Johnson & Johnson shot.
“We’re the group that has been hit the hardest between job losses and child care and scheduling. The ability to not have to go in for two vaccinations was huge,” she said. “And that’s been taken off the table for women, and this is just another thing that shows that women are getting hit yet again.”